or start from open source methods. Learn more about OneLab softwareUse OneLab
Automated ProA Resin Based Antibody Purification

This advanced method offers an end-to-end solution developed around an advanced application, a specialized kit, or an analytical system. It delivers complete functional, ready-to-use protocols that are qualitatively and quantitatively assessed for consistency, executability, and repeatability, usually without needing to make any changes. It operates per batch size and is scalable to accommodate changing requirements.
Overview
Affinity purification of recombinant monoclonal antibody refers to the use of a binding agent to extract an antibody from a complex matrix such as harvested cell culture fluid (HCCF). It is a reversable process where the binding occurs under physiological conditions and the antibody is released by a releasing reagent, typically under acidic conditions. The pH of the final solution is neutralized to maintain the antibody’s stability for long-term storage. In recombinant monoclonal antibody production and analysis, Protein A (ProA) ligand, immobilized on a solid surface, is the most frequently used affinity agent.
In biotherapeutic process optimization, monitoring of process- and product-related quality attributes is routinely performed in a typical BioPharm setting. The Ambr250® parallel bioreactor system is a widely used platform for upstream bioprocess development. In a typical fed-batch experiment, a Design of Experiment (DoE) setup with sampling up to 14 days can generate a large number of samples. To enable time-course analysis of multiple product attributes efficiently and consistently, automated purification becomes a critical part of the overall workflow.
This protocol enables the automated purification of up to 96 HCCF samples using the Andrew+ Pipetting Robot and ProA ligan immobilized on resin in a 96-well plate format. This protocol was utilized in a recent Waters application note3, some of the text of which has been repurposed here. In the data set generated for that application note, an average recovery of 97% ±8% was obtained for 96 HCCF samples at ~1.44 mg/mL concentration. The recovery was determined from peak areas of LC-UV chromatograms collected with a Waters BioResolve Protein A Affinity column, and each sample had an observed %RSD of <1% for its triplicate injections. The distribution of recovery across the 96-well plate is shown in Figure 1.
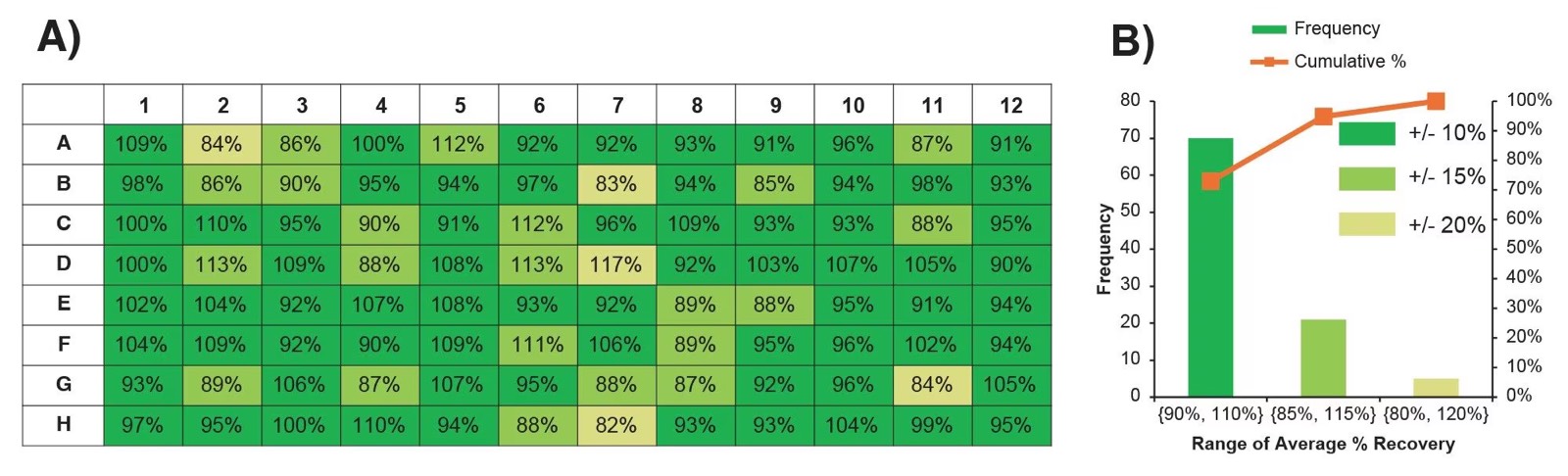
Figure 1: (A) Average mAb recovery per well in 96-well plate following automated ProA purification protocol, n=3. Wells are color-coded according to their value, with dark green values falling within 10% of 100% mAb recovery relative to the unpurified sample (full recovery); light green values falling within 15% of full mAb recovery; and pale yellow falling within 20% of full mAb recovery. (B) Distribution of average mAb recovery in the 96-well plate, following the same color scheme as (A). The cumulative count contributed by each range is represented as a percentage by the orange overlay line. Figure reprinted from “Analytical Scale 96-well Protein A Affinity Resin-Based Purification using Andrew+ Automation Robot Supporting Upstream Bioprocessing” Waters application note (Lit 720009002).
Assay notes
Ensure the ProA resin slurry is maintained at 25% resin by volume for ease of mixing and pipetting. (See “Reagents and Chemicals” section below)
This protocol requires two critical manual actions: sealing the filter plate prior to incubation shaking, and removing the films from the filter plate prior to washing and eluting steps.
- The first plate seal film should be adhered to the bottom of the filter plate prior to the addition of the HCCF samples to the conditioned resin. This will minimize dripping from the filter plate and promote more complete binding to the resin.
- Before the filter plate is placed in the orbital shaker, an additional plate seal film should be adhered to the top of the filter plate to minimize splashing from the resin-sample mixtures.
- Finally, both the top and the bottom plate seal films should be removed once the shaking incubation is complete to allow vacuum filtration and washing steps to proceed.
The recommended volume of Tris and ProA resin are in excess to cover dead volume of the reagent reservoir used. These excess reagents were readily recollected from the reagent reservoir as desired following execution of the protocol.

Figure 2: Andrew+ OneLab deck set up for the ProA-resin purification 96-well plate assay. Samples and reagents locations on Andrew+ deck:
(1-2) Tip Insertion System Domino with 10-300 µL tips, (3) Waters QuanRecovery collection plate in Storage Plate domino, (4) 96 HCCF samples in Waters 350 µL 96-round well plate in Microplate domino, (5) Extraction+ Connected Device manifold, (6) 12-channel reagent reservoir in Deepwell Microplate domino
Reagents and Chemicals
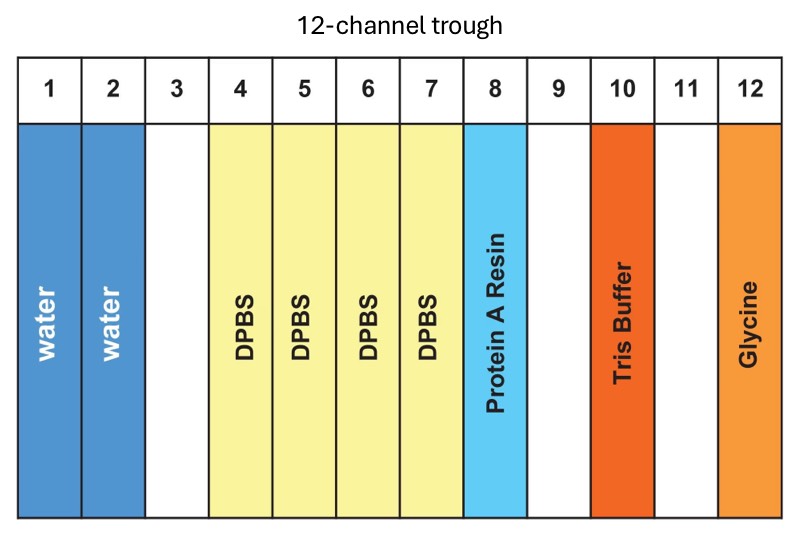
Figure 3: Reagent placement in 12-channel plate. The amount of each reagent is summarized in Table 1. Figure reprinted from “Analytical Scale 96-well Protein A Affinity Resin-Based Purification using Andrew+ Automation Robot Supporting Upstream Bioprocessing” Waters application note (Lit 720009002).
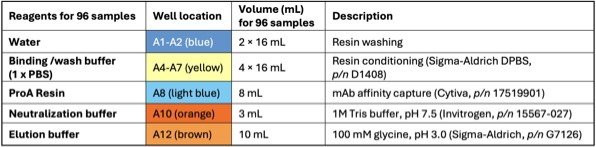
Table 1: Reagent placement in the 12-channel reagent trough.
Instruction for reagent preparation:
- Binding/Wash buffer: 1xDPBS, perform 1:10 dilution of 10xPBS (Ex. Sigma-Aldrich) using H2O.
- ProA Resin: the resin comes as 50% resin in 20% ethanol; following Cytiva’s instruction, centrifuge at 1000g for 3 min, then replace supernatant with 400 mM NaCl in 20% ethanol to 50% resin level. Further dilute with 1xDPBS to 25% resin as the working solution. Thoroughly mix prior to transfer to reagent trough. After each use, mark the liquid level on the tube. In next use, if liquid level is reduced due to evaporation, fill to mark with 20% ethanol, 0.4M NaCl solution.
- Elution buffer: 100 mM glycine, pH adjusted to 3.0 using 5N HCl.
- Neutralization buffer: 1M Tris-HCl, pH 7.5.
Protocols specifications
ProA-resin purification 96-well plate:
- Estimated time of execution: 1 h 17 min
- Hands-on time: 21 min (apply seal to filter plate and mix at 12-14 C for 20 min)
- Tip consumption:
- 136× 5 – 350 µL tips
ProA-resin purification 96-well plate with Shaker+:
- Estimated time of execution: 1 h 21 min
- Hands-on time: 21 min (apply seal to filter plate and mix at 12-14 C for 20 min)
- Tip consumption:
- 136× 5 – 350 µL tips
Ordering information
Andrew+ System Components: Dominos, Devices, Electronic Pipettes & Tips
- Andrew+ Pipetting Robot
- OneLab software
- 2× Tip Insertion System Domino | p/n 186009612
- Extraction+ Base Kit with Plate Gripper | p/n 176005201
- Collection Plate Spacer Kit | p/n 186010527
- Specifically uses the 15mm and 17mm spacers
- Deepwell Microplate Domino | p/n 186009597
- Storage Plate Domino | p/n 186009596
- Microplate Domino | p/n 186009600
- Andrew Alliance Bluetooth Electronic Pipette, 8-ch 300 μL | p/n 186009607
Recommended consumables
- Microplate Shaker+ | p/n 176004577
Additional equipment
- Eppendorf Thermomixer C | p/n Eppendorf 5382000023
- Eppendorf SmartBlock for Microplates and Deepwell Plates | p/n Eppendorf 5363000039
Recommended consumables
- Waters QuanRecovery 700 µL 96-well plate | p/n 186009184
- Waters 350 µL 96-round well collection plate | p/n 186002643
- Sartorius, Optifit Tips, 5-350 µL, 10pk refill | p/n 700013297
- Axygen 12-channel trough | p/n Corning RES-MW12-HP
- AcroPrep Advanced 350 µL 96-well filter plate 10K membrane | p/n Pall or Cytiva 8019
References
- Stephan M. Koza, Caitlin M. Hanna, Albert H. W. Jiang, Ying Qing Yu, Automated High-Throughput Analytical-Scale Monoclonal Antibody Purification Using Production-Scale Protein A Affinity Chromatography Resin. Waters Application Note. No. 720007861, February 2023.
https://www.waters.com/nextgen/us/en/library/application-notes/2023/automated-high-throughput-analytical-scale-monoclonal-antibody-purification-using-production-scale-protein-a-affinity-chromatography-resin.html. - Yun W Alelyunas, Julie Wushensky, Mark Wrona, Rui Chen, Analytical Scale 96-well Protein A Affinity Resin-Based Purification using Andrew+™ Automation Robot Supporting Upstream Bioprocessing. Waters Application Note. No. 720009002, September 2025.
https://www.waters.com/nextgen/us/en/library/application-notes/2025/analytical-scale-96-well-protein-a-affinity-resin-based-purification-using-andrew-automation-robot-supporting-upstream-bioprocessing.html. - MabSelect™ antibody purification chromatography resin. Cytiva. MabSelect™ antibody purification chromatography resin. Cytiva.
Protocols


Contact info

 This is an open access protocol distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
This is an open access protocol distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. 